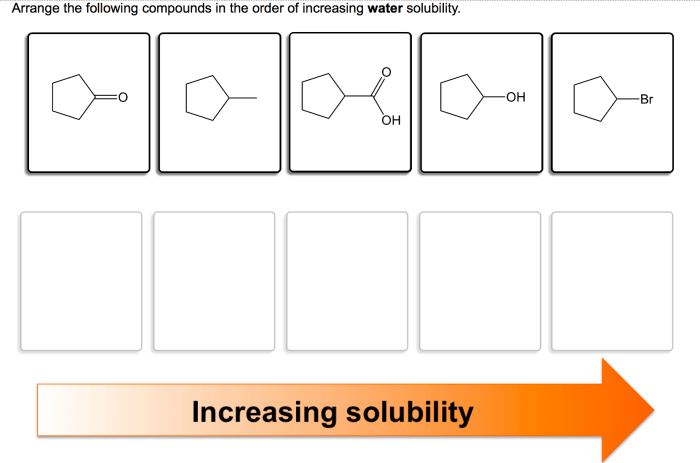
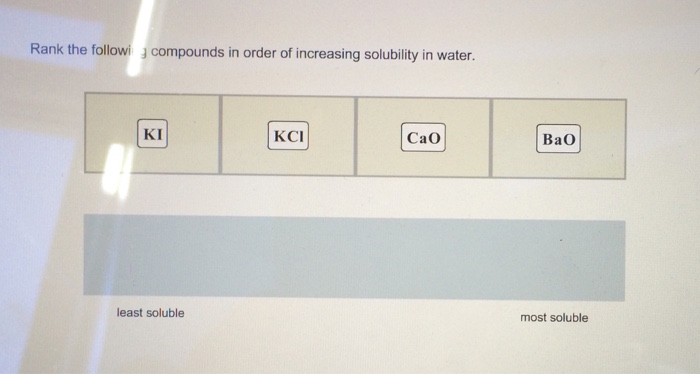
Rank the compounds in order of increasing water solubility – Embark on a journey to unravel the secrets of water solubility, a fundamental property that governs the behavior of compounds in aqueous environments. As we delve into the intricacies of polarity, hydrogen bonding, and experimental methods, we will illuminate the factors that dictate the solubility of compounds and explore their profound implications in diverse fields.
Join us as we embark on a quest to rank compounds in order of their increasing water solubility, unlocking the mysteries that lie at the heart of this captivating scientific endeavor.
Factors Affecting Water Solubility
The solubility of a compound in water depends on various factors, including its polarity, molecular size, and the presence of functional groups capable of forming hydrogen bonds.
Polarity, Rank the compounds in order of increasing water solubility
Polarity refers to the uneven distribution of electrons within a molecule, creating a partial positive or negative charge on different atoms. Polar compounds, such as ionic compounds or those with polar functional groups, are more soluble in water because they can interact with the polar water molecules through dipole-dipole interactions or ion-dipole interactions.
Examples of polar compounds include sodium chloride (NaCl), ethanol (CH 3CH 2OH), and ammonia (NH 3).
Hydrogen Bonding
Hydrogen bonding is a strong dipole-dipole interaction that occurs between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom. Hydrogen bonding significantly enhances water solubility because it forms additional attractive forces between the solute and water molecules.
Examples of compounds that can form hydrogen bonds with water include alcohols (R-OH), carboxylic acids (R-COOH), and amides (R-CONH 2).
Experimental Methods for Determining Water Solubility
Several experimental methods can be used to determine the water solubility of a compound, including the shake-flask method, HPLC or GC, and UV-Vis spectroscopy.
Shake-flask Method
The shake-flask method is a simple and widely used technique. It involves adding an excess of the compound to a known volume of water and shaking the mixture vigorously. The undissolved compound is then filtered or centrifuged, and the concentration of the dissolved compound in the filtrate is determined using analytical techniques such as HPLC or UV-Vis spectroscopy.
HPLC or GC
High-performance liquid chromatography (HPLC) or gas chromatography (GC) can be used to separate and quantify the dissolved compound in the filtrate obtained from the shake-flask method. These techniques provide accurate and precise measurements of water solubility.
UV-Vis Spectroscopy
UV-Vis spectroscopy can be used to determine the water solubility of compounds that absorb light in the ultraviolet or visible region. The absorbance of a solution is directly proportional to the concentration of the dissolved compound. By measuring the absorbance of a series of solutions with known concentrations, a calibration curve can be constructed, which can then be used to determine the concentration of the dissolved compound in the filtrate.
Essential Questionnaire: Rank The Compounds In Order Of Increasing Water Solubility
What is the significance of water solubility in drug design?
Water solubility plays a crucial role in drug design as it influences the bioavailability, distribution, and elimination of drugs within the body. Understanding the water solubility of drug candidates is essential for optimizing their efficacy and minimizing potential adverse effects.
How does water solubility impact environmental chemistry?
Water solubility is a key factor in determining the fate and transport of chemicals in the environment. It influences the partitioning of compounds between water and soil, air, and biota, affecting their persistence, bioavailability, and potential toxicity.