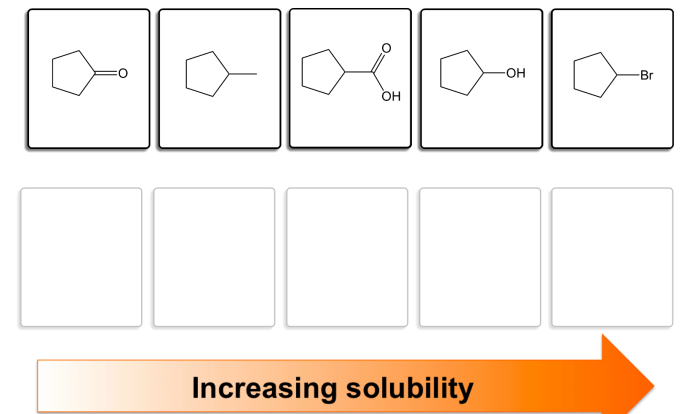
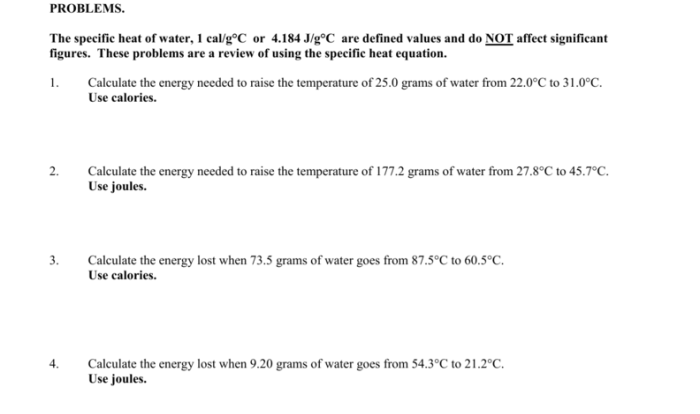
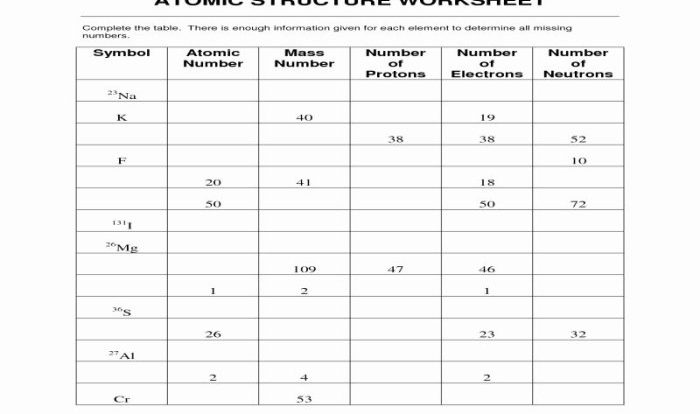
Acid base reactions worksheet answers unveil the intricacies of acid-base reactions, empowering learners with a profound understanding of their fundamental principles and diverse applications. This comprehensive guide delves into the heart of acid-base chemistry, providing a panoramic view of its theoretical underpinnings and practical implications.
Embark on a journey of discovery as we explore the types of acid-base reactions, unravel the concepts of pH and pOH, and delve into the significance of dissociation constants. Our in-depth analysis sheds light on the role of acid-base reactions in everyday life, industries, and environmental processes.
Introduction
Acid-base reactions are chemical reactions that involve the transfer of protons (H+) between reactants.
These reactions are crucial in many biological and industrial processes, including digestion, photosynthesis, and the production of fertilizers.
Importance of Acid-Base Reactions
- Acid-base reactions play a vital role in maintaining the pH balance of living organisms.
- They are essential for the proper functioning of enzymes and other proteins.
- Acid-base reactions are used in a wide variety of industrial processes, such as the production of fertilizers, dyes, and pharmaceuticals.
Types of Acid-Base Reactions
Acid-base reactions are chemical reactions that involve the transfer of protons (H+ ions) between reactants. These reactions are classified into three main types: neutralization reactions, precipitation reactions, and gas evolution reactions.
Neutralization Reactions, Acid base reactions worksheet answers
Neutralization reactions occur between an acid and a base, resulting in the formation of a salt and water. The salt is an ionic compound composed of the positively charged cation from the base and the negatively charged anion from the acid.
Water is a neutral compound with the formula H2O.For example, the neutralization reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water:“`HCl + NaOH → NaCl + H2O“`
Precipitation Reactions
Precipitation reactions occur between two soluble ionic compounds in solution, resulting in the formation of an insoluble solid precipitate. The precipitate is a compound that has a low solubility in the solvent and forms a solid phase.For example, the precipitation reaction between silver nitrate (AgNO3) and sodium chloride (NaCl) produces silver chloride (AgCl) as a white precipitate:“`AgNO3 + NaCl → AgCl(s) + NaNO3“`
Gas Evolution Reactions
Gas evolution reactions occur when a chemical reaction produces a gas as one of the products. The gas escapes from the reaction mixture as bubbles, resulting in an increase in volume.For example, the reaction between hydrochloric acid (HCl) and sodium carbonate (Na2CO3) produces carbon dioxide (CO2) gas, which escapes from the solution as bubbles:“`
HCl + Na2CO3 → 2NaCl + H2O + CO2(g)
“`
Acid-Base Strength
The strength of an acid or base is determined by its ability to donate or accept protons (H+ ions). Strong acids and bases completely dissociate in water, releasing all of their protons or hydroxide ions (OH- ions), respectively. Weak acids and bases only partially dissociate in water, releasing only a small fraction of their protons or hydroxide ions.
pH and pOH
The pH of a solution is a measure of its acidity or basicity. It is defined as the negative logarithm of the hydrogen ion concentration ([H+]). The pOH of a solution is a measure of its basicity. It is defined as the negative logarithm of the hydroxide ion concentration ([OH-]).
The pH and pOH of a solution are related by the following equation:
pH + pOH = 14
This equation shows that a solution with a low pH (high acidity) will have a high pOH (low basicity), and vice versa.
Strong and Weak Acids and Bases
Strong acids are those that completely dissociate in water, releasing all of their protons. Examples of strong acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3).
Weak acids are those that only partially dissociate in water, releasing only a small fraction of their protons. Examples of weak acids include acetic acid (CH3COOH), carbonic acid (H2CO3), and hydrofluoric acid (HF).
Strong bases are those that completely dissociate in water, releasing all of their hydroxide ions. Examples of strong bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2).
Weak bases are those that only partially dissociate in water, releasing only a small fraction of their hydroxide ions. Examples of weak bases include ammonia (NH3), pyridine (C5H5N), and sodium bicarbonate (NaHCO3).
Dissociation Constants
The dissociation constant (Ka) of an acid is a measure of its strength. It is defined as the equilibrium constant for the dissociation of the acid in water.
The larger the Ka value, the stronger the acid. Strong acids have Ka values that are much greater than 1, while weak acids have Ka values that are much less than 1.
The dissociation constant (Kb) of a base is a measure of its strength. It is defined as the equilibrium constant for the dissociation of the base in water.
The larger the Kb value, the stronger the base. Strong bases have Kb values that are much greater than 1, while weak bases have Kb values that are much less than 1.
Applications of Acid-Base Reactions: Acid Base Reactions Worksheet Answers
Acid-base reactions have numerous applications in everyday life, industries, and environmental processes.
In everyday life, acid-base reactions are involved in various processes, such as:
- Digestion: Stomach acid (hydrochloric acid) helps break down food.
- Battery operation: Acid-base reactions generate electricity in batteries.
- Cleaning: Acids and bases are used in cleaning products to remove dirt and stains.
In industries, acid-base reactions are crucial for:
- Fertilizer production: Acid-base reactions are used to produce fertilizers, such as ammonium nitrate and superphosphate.
- Textile manufacturing: Acids and bases are used to treat and dye fabrics.
- Paper production: Acid-base reactions are involved in the bleaching and sizing of paper.
Acid-base reactions also have environmental implications:
- Acid rain: Emissions from factories and vehicles can lead to the formation of acid rain, which damages forests and aquatic ecosystems.
- Water pollution: Industrial wastewater and agricultural runoff can contain acids or bases that can contaminate water bodies.
- Carbon dioxide absorption: Oceans absorb carbon dioxide from the atmosphere, which forms carbonic acid and lowers the pH of seawater.
Worksheet Questions and Answers
This section provides a comprehensive table of acid-base reaction questions and answers, covering various aspects of acid-base chemistry.
The table includes questions on identifying acid-base reactions, predicting products, and calculating pH, enabling students to assess their understanding of these fundamental concepts.
Identifying Acid-Base Reactions
- Question:Which of the following reactions is an acid-base reaction?
- Answer:NaOH + HCl → NaCl + H 2O
- Question:Identify the acid and base in the reaction: H 2SO 4+ NaOH → Na 2SO 4+ H 2O
- Answer:Acid: H 2SO 4; Base: NaOH
Predicting Products
- Question:Predict the products of the reaction between CH 3COOH and KOH.
- Answer:CH 3COOK + H 2O
- Question:Write the balanced equation for the reaction between HNO 3and NH 3.
- Answer:HNO 3+ NH 3→ NH 4NO 3
Calculating pH
- Question:Calculate the pH of a 0.1 M solution of HCl.
- Answer:pH = 1
- Question:A solution has a pH of 12. What is the concentration of OH –ions in the solution?
- Answer:[OH –] = 10 -2M
Practice Problems
Practice problems on acid-base reactions enhance understanding of neutralization, precipitation, and gas evolution reactions. These problems provide hands-on experience in applying concepts and balancing chemical equations.
Neutralization Reactions, Acid base reactions worksheet answers
Neutralization reactions involve the reaction of an acid and a base to form salt and water. The following practice problems focus on balancing and predicting the products of neutralization reactions:
- Balance the following neutralization reaction: HCl + NaOH →
- Predict the products of the neutralization reaction between sulfuric acid (H 2SO 4) and potassium hydroxide (KOH).
Precipitation Reactions
Precipitation reactions occur when two solutions containing ions combine to form an insoluble solid precipitate. Practice problems on precipitation reactions emphasize predicting the formation of precipitates and writing balanced chemical equations:
- Predict the precipitate formed when aqueous solutions of barium chloride (BaCl 2) and sodium sulfate (Na 2SO 4) are mixed.
- Balance the following precipitation reaction: FeCl 3+ NaOH →
Gas Evolution Reactions
Gas evolution reactions involve the production of a gas as one of the products. Practice problems on gas evolution reactions focus on identifying the gas produced and balancing chemical equations:
- Identify the gas produced when hydrochloric acid (HCl) reacts with calcium carbonate (CaCO 3).
- Balance the following gas evolution reaction: HNO 3+ NaHCO 3→
Clarifying Questions
What are acid-base reactions?
Acid-base reactions are chemical reactions involving the transfer of protons (H+) between species, resulting in the formation of new substances.
What is the importance of acid-base reactions?
Acid-base reactions play a crucial role in various biological, industrial, and environmental processes, such as digestion, manufacturing, and water purification.
What is pH?
pH is a measure of the acidity or basicity of a solution, ranging from 0 to 14, with 7 representing neutrality.
What is the difference between a strong acid and a weak acid?
Strong acids dissociate completely in water, releasing all their protons, while weak acids dissociate partially.